You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
MNKYPOX Mpox (Monkeypox) clade 1b International Emergency Thread
- Thread starter phloydius
- Start date
psychgirl
TB Fanatic
Heliobas Disciple
TB Fanatic

Potential single-dose smallpox and mpox vaccine moves forward
Vaccines that prevent smallpox and mpox come in two varieties. One uses a single shot of a live virus but carries the risk of serious side effects; the other, which is newer and made with replication deficient virus, has fewer side effects but requires two doses.
Potential single-dose smallpox and mpox vaccine moves forward
by American Society for Microbiology
November 13, 2024
Vaccines that prevent smallpox and mpox come in two varieties. One uses a single shot of a live virus but carries the risk of serious side effects; the other, which is newer and made with replication deficient virus, has fewer side effects but requires two doses.
An experimental vaccine under development at Tonix Pharmaceuticals in Frederick, Md., aims to combine the benefits of both vaccine strategies. It uses the horsepox virus as a protective agent to confer the safety of a multi-dose vaccine in a single shot.
In mSphere, scientists at Tonix Pharmaceuticals report on studies suggesting that the horsepox virus in the experimental vaccine is substantially more attenuated—and thus less likely to trigger a systemic infection—than the vaccinia virus used in the single-dose vaccine already approved by the U.S. Food and Drug Administration (FDA).
"We are trying to go for the immunity of the old vaccines and safety profile of the new ones, and so far the data are very promising," said virologist Farooq Nasar, Ph.D, senior author on the study. "We would like to be in the middle."
Smallpox, which is caused by the variola virus, was a highly contagious disease with a fatality rate between 30% and 97%. Historical records suggest it began infecting people thousands of years ago, and the World Health Organization (WHO) estimated that smallpox killed between 300-500 million people in the 20th century. An effective vaccination campaign that began in the 1950s stopped the spread, and in 1980 the WHO declared the disease eradicated.
Mpox, formerly known as monkeypox, is a related disease caused by the monkeypox virus, which, like variola, is a member of the genus Orthopoxvirus. Multiple mpox outbreaks have occurred since 2022 and estimates of case fatality rate vary from 0.2% to 11%. The two FDA-approved vaccines for smallpox are also approved for use to prevent mpox infection.
The approved live-virus smallpox vaccine includes the vaccinia virus, a less harmful member of the Orthopoxvirus family that helps the body develop defenses against smallpox infection. A few years ago, however, researchers discovered that smallpox vaccines used before and during the American Civil War contained a virus more than 99% genetically similar to horsepox—another Orthopoxvirus.
That discovery launched investigations into whether a vaccine with live, attenuated horsepox could confer immunity without the unwelcome side effects of the live vaccinia virus-based vaccine.
Previous studies by researchers at Tonix Pharmaceuticals and elsewhere have reported that the experimental vaccine provoked an antibody response but did not cause disease in non-human primates; in addition, the vaccine protected non-human primates against lethal exposure to the monkeypox virus with no occurrence of clinical disease.
The new study, led by virologists Nasar and Stefanie Trefry, Ph.D., builds on those findings. Testing in both human cell lines and mouse models, the researchers compared virus replication in models with vaccinia virus strains to those given the experimental horsepox virus.
They found that the horsepox virus is attenuated up to 1,000-fold more than the vaccinia virus strains, which means it contained a much lower concentration of infectious particles. Attenuation weakens a virus, which means it is less likely to trigger a systemic infection in the host.
Mice given the vaccinia-based vaccine strains often developed severe symptoms, while mice given the experimental horsepox vaccine showed no adverse effects.
The researchers are now planning phase I human clinical trials to test the safety of the vaccine in people. Smallpox now only exists in laboratories in the United States and Russia, and researchers continue to investigate smallpox vaccines as a defense against smallpox as a bioterror agent.
The more urgent public health concern right now, said Nasar, is preventing future mpox outbreaks. The new vaccine hopes to serve both purposes.
"We're focused on making a product that can be used against both mpox and smallpox," he said.
More information: mSphere (2024). DOI: 10.1128/msphere.00265-24
Provided by American Society for Microbiology
LightEcho
Has No Life - Lives on TB
Maybe we should just eliminate the research labs and then there is no small pox threat. Oh, but that would also eliminate the "need" for more poison vaxxes.Smallpox now only exists in laboratories in the United States and Russia,
desertvet2
Has No Life - Lives on TB
Meemur
Voice on the Prairie
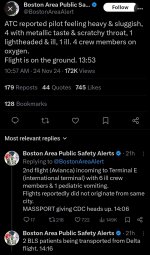
Merde.Clade 1b is in California.
And the Thanksgiving travel season is less than 9 to 10 days away. Maybe I can sneak in another professional haircut and a teeth cleaning in before Clade 1b starts running rampant in Iowa.
helen
Panic Sex Lady
Location.
2:26
New mpox strain reported in San Mateo County
View: https://youtu.be/oPMx6xZYTlM?feature=shared
2:26
New mpox strain reported in San Mateo County
Damn! This is gonna end up making me drag out my half finished recipe for a Monkeypox topical treatment...probably a spray.
I was so hoping to not need it!
Summerthyme
I was so hoping to not need it!
Summerthyme
helen
Panic Sex Lady
Do you think zinc oxide would at least prevent secondary bacterial infection in the lesions?Damn! This is gonna end up making me drag out my half finished recipe for a Monkeypox topical treatment...probably a spray.
I was so hoping to not need it!
Summerthyme
Baby butt paste.
Very likely, it will at least sooth and protect, as well as dry the lesions. Colloidal silver rinses, as well as Betadine (neither stings, but Betadine stains... which does give the benefit of knowing which spots were treated.Do you think zinc oxide would at least prevent secondary bacterial infection in the lesions?
Baby butt paste.
I'd probably choose the silver for most treatments, simply because it's milder.
Summerthyme
Heliobas Disciple
TB Fanatic
Clade 1b is in California.
htt ps://ww w.c dc. gov/media/releases/s1116-california-first-clade.html
(fair use applies)
California confirms first clade I mpox case
Statement
For immediate release: November 16, 2024
CDC to receive samples for addition viral characterization
The California Department of Public Health confirmed, through laboratory testing, the first known case of clade I mpox in the United States. This case is related to an ongoing outbreak of clade I mpox in Central and Eastern Africa. The risk of clade I mpox to the public remains low, and there continue to be sporadic clade II mpox cases in the United States.
The case was diagnosed in a person who recently traveled from Eastern Africa. The individual was treated shortly after returning to the United States at a local medical facility and released. Since then, the person has isolated at home, is not on treatment specific for mpox, and symptoms are improving. Based on their travel history and symptoms, patient specimens were tested and confirmed for the presence of clade I monkeypox virus. Specimens are being sent to CDC for additional viral characterization. Additionally, CDC is working with the state to identify and follow up with potential contacts.
Casual contact, like you might have during travel, is unlikely to pose significant risks for transmission of mpox. While investigations continue into this case, CDC guidance has not changed. Protect yourself from mpox by:
- Avoiding close contact with people who are sick with symptoms of mpox, including those with skin or genital lesions
- Avoiding contact with contaminated materials used by people who are sick (such as clothing, bedding, toothbrushes, sex toys, or materials used in healthcare settings)
- And if you're eligible, get both recommended doses of mpox vaccine.
People with mpox often get a rash that may be located on hands, feet, chest, face, mouth and/or near the genitals, including penis, testicles, labia, vagina, and anus. The incubation period is 3–17 days. During this time, a person does not have symptoms and may feel fine.
The anticipated overall risk of clade I mpox to the general population in the United States from the outbreak in Central and Eastern Africa is low. Earlier this year CDC conducted a risk assessment which included epidemiologic data from Central and Eastern Africa, data from the ongoing mpox outbreak in the United States caused by clade IIb, and historical data on clade I mpox outbreaks in DRC and other affected countries. In addition, CDC has simulated clade I mpox outbreaks. These simulations indicate that close-contact transmissions within and between households are unlikely to result in a large number of mpox clade I cases in the United States. Additionally, in Sweden, Thailand, Germany, and India there was no apparent onward spread of the virus and the onward spread in the United Kingdom has been limited to close, household contacts so far.
CDC continues to work in Central and Eastern Africa to help stop mpox transmission at the source. This ongoing work includes laboratory training, supplies for diagnostic testing (including genetic sequencing), training of frontline health and epidemiologic workers, support for surveillance in people and animals, support for infection prevention and control, risk communication and community engagement, and direct technical assistance in outbreaks, as well as research collaborations.
CDC has more than two years of experience responding to mpox in the United States due to the ongoing 2022 global clade II mpox outbreak and has adjusted existing domestic public health systems and structures to respond to any outbreak of clade I mpox in the United States. CDC issued guidance for travelers to countries in Central and Eastern Africa experiencing outbreaks earlier this year. CDC continues to recommend that clinicians request expedited clade specific testing for suspect clade I mpox cases with travel history to Central and Eastern Africa. CDC is also helping communities monitor the presence of both clades of mpox virus in wastewater samples. Data from samples can provide an early warning of mpox activity and spread in communities. CDC combines wastewater data with other data to decide if there is a need for further testing or other actions in collaboration with state and local public health partners.
More information on mpox is available online at Mpox | Mpox | CDC.
psychgirl
TB Fanatic
Good idea.Merde.
And the Thanksgiving travel season is less than 9 to 10 days away. Maybe I can sneak in another professional haircut and a teeth cleaning in before Clade 1b starts running rampant in Iowa.
I’d sneak in a lot of OTHER things too, before Christmas.
Damn! This is gonna end up making me drag out my half finished recipe for a Monkeypox topical treatment...probably a spray.
I was so hoping to not need it!
I too have mixed up a little something for MPox but unlike you I am only guessing at what will work!
Summerthyme
Damn! This is gonna end up making me drag out my half finished recipe for a Monkeypox topical treatment...probably a spray.
I was so hoping to not need it!
Summerthyme
I too have a mixture ready but unlike you I don't really know what I am doing!
Neither do I! I've never treated pox, and never wanted to! But I figure we need both drying and emollient qualities, with lysine and some anti-viral essential oils, should help with symptoms and help speed healing.I too have a mixture ready but unlike you I don't really know what I am doing!
Summerthyme
My thoughts exactly! I am just unsure of proportions! But something is better than nothing. My hubby has never been vaxxed for small pox and lots of other things! Gotta love rural Alabama.Neither do I! I've never treated pox, and never wanted to! But I figure we need both drying and emollient qualities, with lysine and some anti-viral essential oils, should help with symptoms and help speed healing.
Summerthyme
Things to replenish: colloidal silver, oil of oregano, sarracenia purpurea. What else?
lomatium I think that is the correct spelling. Was used by indians to treat smallpox I think but is an antiviral.
I have no way of knowing whether lysine will work on pox. But I was once asked to see a neighbor boy (about 8) who had, as it turned out, a herpes virus sore in his eye. Fortunately, it was on the white of the eye, but it still could have spread and posed serious danger to his vision.Lesions occur inside the mouth, nose, and throat, which makes eating nearly impossible. I don't know what do about the eyes, but hopefully real medical care won't be impossible.
I still think it's just another excuse for lockdowns and not very likely for most of us to get.
Since they refused to go to a doctor for something "minor", I mixed L-lysine in some distilled water, put it in a sterile dropper bottle, and had them treat the eye 4x a day.
In 3 days, the herpes lesion was gone.
For the mouth and throat, I'd add L-lysine to some salt water (1/2 tsp to 1 cup warm water). Then rinse, hold in the mouth for 30 seconds and spit out. Repeat, but gargle it deep in your throat.
Summerthyme
Tigerlily
Veteran Member
SpikenardThings to replenish: colloidal silver, oil of oregano, sarracenia purpurea. What else?
I have no way of knowing whether lysine will work on pox. But I was once asked to see a neighbor boy (about 8) who had, as it turned out, a herpes virus sore in his eye. Fortunately, it was on the white of the eye, but it still could have spread and posed serious danger to his vision.
Since they refused to go to a doctor for something "minor", I mixed L-lysine in some distilled water, put it in a sterile dropper bottle, and had them treat the eye 4x a day.
In 3 days, the herpes lesion was gone.
For the mouth and throat, I'd add L-lysine to some salt water (1/2 tsp to 1 cup warm water). Then rinse, hold in the mouth for 30 seconds and spit out. Repeat, but gargle it deep in your throat.
Summerthyme
When I had a particularly bad case of shingles I used lemon balm tincture mixed in a bit of water and swished and spat out. I found it very soothing. I mixed it with many other herbs so I don't know what did the trick but in 3 days I was sipping and by day 4 I was eating. Someone here Moldy, recommended that to me. And it worked!
helen
Panic Sex Lady
Escaped primates open can of worms for South Carolina's Alpha Genesis research lab
Escaped primates open can of worms for South Carolina's Alpha Genesis research lab
abcnews.go.com
With four primates still on the loose after 43 of them escaped on Nov. 6 from the Alpha Genesis Inc. research laboratory in South Carolina, the Low Country facility has come under intense scrutiny.
Animal rights groups have cited the company's history of violations and previous monkey breakouts; a member of Congress has called for an inquiry into its oversight by multiple federal agencies; and residents voiced concern the furry fugitives might spread disease throughout their community.
On top of it all, Alpha Genesis founder and CEO Gregory Westergaard told ABC News his company is investigating whether the release of the monkeys was "an intentional act" by an employee.
The quest for freedom by the pack of young female rhesus macaques coincides with the rapid expansion of the 100-acre Alpha Genesis facility and is casting light on a disruption in the U.S. medical research industry that sounds like a plot for a science fiction thriller. A 2023 report sponsored by the National Institutes of Health warned of a crisis involving the Chinese government that "undermines the security of the nation’s biomedical research enterprise."
The case of the absconding primates has also raised questions about why the amount of federal contracts received by the testing and breeding operation has jumped more than 160% since 2021. According to USASpending.gov, a government website that tracks federal spending, the company has been granted $19 million in federal contracts this year alone.
"It’s shocking how much money is being spent on testing primates," Rep. Nancy Mace, R-S.C., told ABC News.
Mace's district encompasses the Beaufort County community of Yemassee, where the 6,701 primates housed at the sprawling Alpha Genesis facility nearly triple the number of town residents.
In a formal letter to the NIH, the agency that funds laboratory research, and the U.S. Department of Agriculture, which inspects and regulates breeding facilities, Mace expressed "very urgent concerns regarding federal oversight of Alpha Genesis." Mace said the prolonged attempts to recapture all of the primates are "placing the animals and my constituents at risk."
"A lot of constituents were concerned about whether or not the primates that escaped were sick or ill, or have been tested on," Mace told ABC News. "There were a lot of folks concerned about the facility being a breeding facility and the testing that goes on there as well."
The incident some locals have referred to as "the great escape" has illuminated the international crisis hitting the animal research industry that Alpha Genesis' Westergaard said has become "an issue of national security."
In 2020, the Chinese government, the world's primary breeder of research monkeys, banned the exports of nonhuman primates (NPH) to labs in the United States and elsewhere, triggering an international shortage of the animals just as research scientists were scrambling to come up with vaccines to combat the COVID-19 pandemic, according to a May 2023 report by National Academy of Engineering and National Academy of Medicine.
Primates, according to the NIH-supported report, are valuable in answering certain research questions because of their genetic, anatomic, physiologic and behavioral similarities to humans. However, the China ban on exporting research animals exacerbated the shortage and stalled NIH-funded research, according to the report.
Alpha Genesis federal contracts pre and post-pandemic
The report concluded that the United States "needs to prioritize expansion" of domestic primate breeding programs.
"Relying on importing these animals from other countries is unsustainable, and dependence on international sources undermines the security of the nation’s biomedical research enterprise," the report warned.
In 2021, the National Primate Research Centers could not meet two-thirds of researcher requests for rhesus macaques, according to the report.
"Researchers also face increased wait times for animals, and costs have risen 10% to 200% for a single animal, depending on the species," the report said.
The crisis prompted Alpha Genesis to increase its domestic breeding of research primates. According to Rep. Mace, the company also manages the NIH's so-called "Monkey Island" on Morgan Island in Beaufort County, which holds another 3,300 primates.
Westergaard told ABC News that Alpha Genesis employs 275 people, plus 30 or so contractors.
In addition to breeding lab monkeys, Alpha Genesis provides researchers across the country with biological products and materials, including serum, plasma, whole blood and tissue samples from a wide variety of research species, according to the company's website. The private company's researchers have helped develop several therapeutic drugs and vaccines, including those to treat the COVID-19 virus.
Price of Research Monkeys
Alpha Genesis lab, Adobe stock
According to NIH online records provided to USASpending.gov, the crisis appears to be in accord with a boost in federal contracts Alpha Genesis has received, jumping from $7.3 million in 2021 to $12.3 million in 2022, $14.2 million in 2023 and $19 million this year.
"The price of research monkeys has indeed increased a great deal since the Chinese banned all exports," Westergaard said in an email to ABC News. "Prior to the ban monkeys sold for around $4K - $6K, after the ban prices have increased to $10K - $30K+ due to increased costs of raising animals in the US compared to China. An important point to note is that the shortage remains severe and a great deal of research in the US simply cannot be done because animals are not available at any cost."
Westergaard said some suppliers of laboratory primates have turned to the illegal sourcing of wild-caught monkeys from Cambodia, "which we have not done."
Forty-three rhesus macaque monkey escaped from the Alpha Genesis research lab Castle Hall Road in Yemassee, South Carolina, Nov. 07, 2024.
Yemassee Police Department
"It should also be noted that the Chinese government is seeking worldwide domination in medical research and the development of bio-weapons to target US citizens and our allies," Westergaard said.
He added, "Alpha Genesis is a leading provider of NHPs to the US market and has been instrumental in attempting to fill this void. The alternative is to allow the Chinese to dominate medical development to the severe detriment of our National Security interests."
Asked by ABC News whether the rapid expansion of Alpha Genesis' breeding and testing operations might have played a role in the escape of the 43 primates, Westergaard said the cause of the escape remains under investigation, including whether it was the result of "human error" or an "intentional act."
"All the information we have thus far indicates that this is human error due to an employee failing to secure containment doors behind her, and a third door directly containing the animals, while doing routine cleaning and feeding," Westergaard said in an email. "The enclosure was brand new and in perfect working order. We continue to investigate in an attempt to determine to the greatest extent possible whether this was or was not an intentional act."
Westergaard said that immediately after the incident occurred, the employee’s supervisor told her she could be fired if it was determined that no structural failure of the primates' enclosure led to the incident. Westergaard said the employee walked off the job and has not returned.
As of early Tuesday, four of the escaped primates remained on the loose, Westergaard said. Two were caught Monday, he said.
"The girls from today are in good health and the others continue to thrive," Westergaard said Monday. "We believe the four monkeys remaining are probably all together either in the area adjacent to our property or somewhere else very close by."
Mace has requested answers from NIH Director Monica Bertagnolli, USDA Deputy Administrator for Animal Care Sarah Helming, and Acting Director Axel Wolf of the NIH's Office of Laboratory Animal Welfare. She also noted that this was not the first time problems had arisen at the research lab.
Mace, who told ABC News she is against animal testing, cited in her letter a September 2022 USDA inspection report of Alpha Genesis that found six separate incidents of animals escaping from their primary enclosures between January and August of 2022. Mace also cited escapes dating back to 2014, when more than two dozen monkeys slipped out of the facility, resulting in a fine from the USDA.
The 2022 USDA inspection report, which ABC News reviewed, also found an infant monkey died after becoming entangled in a stretch of gauze material used in an enclosure to hold a water bottle; said two primates were found dead in their enclosures with their fingers entrapped in structures inside their cages; and documented that one animal died from trauma and four others required veterinary care after they were placed in incorrect enclosures and were attacked by other primates unfamiliar with them.
As a result of the inspection, Alpha Genesis, according to the report, took corrective action to secure enclosures and "made significant changes" to avoid putting primates in the wrong enclosures.
The latest USDA routine inspection of Alpha Genesis lab was conducted on May 21 and concluded, "No non-compliant items identified during this inspection," according to USDA online records.
"This is also true of several other inspections in recent years," Westergaard said. "For a facility of this size that is quite remarkable."
Mace said she met with Westergaard last week to discuss the escape and what Alpha Genesis is doing to round up the monkeys.
“It was an interesting conversation," Mace said. "He tried to tell me how good the primates have it at his facility. And my response was, they have it good until you kill them with disease."
Asked about the conversation, Westergaard responded: "I spoke to the congresswoman last week and at that time she said that she recognized the economic importance of our company to the people of the Low Country and that as a locally-owned business, she would continue to offer her full support."
Angela Grimes, CEO of Born Free USA, an international wildlife conservation and animal protection organization, told ABC News that her group has sent a letter to Alpha Genesis offering to rehome the escaped primates to its animal sanctuary in South Texas, where more than 200 rhesus macaque monkeys now reside, including some rescued from U.S. research labs. She said an anonymous donor has pledged $250,000 to help move the animals to the sanctuary.
"What we’d like to see is these animals be released to the Born Free USA sanctuary in South Texas, where they can have some of that freedom that they’ve just gotten a taste of," Grimes said.
Angela Grimes, the CEO of the animal rights group Born Free USA.
Grimes said Alpha Genesis has not responded to her group's offer.
The nonprofit Born Free USA Primate Sanctuary, a 175-acre facility in Dilley, Texas, has been accredited since 2009 by the Global Federation of Animal Sanctuaries, GFAS executive director Valerie Taylor told ABC News, adding that her group conducts rigorous assessments and inspections of animal sanctuaries across the United States and around the globe to ensure the highest standard of animal care possible. Taylor said U.S. animal sanctuaries undergo accreditation every three years and that her organization recently visited the Born Free USA sanctuary as part of the reaccreditation process.
"We meet and exceed GFAS’ highest standards," Grimes said.
Grimes said the medical research industry needs to research alternatives to subjecting primates to experimental testing of deadly diseases.
"I understand human health is important, but I also look at the other viable alternatives that are out there that do not result in the suffering and death of animals," Grimes said.
Westergaard said testing of primates is necessary, though.
"There is no safe or effective way to make the leap from simpler model organisms like mice and rats to humans without using NHPs as an intermediary," Westergaard said. "The therapeutics created using NHPs as research models directly lead to lifesaving and life-prolonging treatments and cures for human disease. Without NHPs as a research model, the world would still be ravaged with wide-spread polio, smallpox wouldn’t be eradicated, and HIV would still be a death sentence."
Escaped primates open can of worms for South Carolina's Alpha Genesis research lab
abcnews.go.com
With four primates still on the loose after 43 of them escaped on Nov. 6 from the Alpha Genesis Inc. research laboratory in South Carolina, the Low Country facility has come under intense scrutiny.
Animal rights groups have cited the company's history of violations and previous monkey breakouts; a member of Congress has called for an inquiry into its oversight by multiple federal agencies; and residents voiced concern the furry fugitives might spread disease throughout their community.
On top of it all, Alpha Genesis founder and CEO Gregory Westergaard told ABC News his company is investigating whether the release of the monkeys was "an intentional act" by an employee.
The quest for freedom by the pack of young female rhesus macaques coincides with the rapid expansion of the 100-acre Alpha Genesis facility and is casting light on a disruption in the U.S. medical research industry that sounds like a plot for a science fiction thriller. A 2023 report sponsored by the National Institutes of Health warned of a crisis involving the Chinese government that "undermines the security of the nation’s biomedical research enterprise."
The case of the absconding primates has also raised questions about why the amount of federal contracts received by the testing and breeding operation has jumped more than 160% since 2021. According to USASpending.gov, a government website that tracks federal spending, the company has been granted $19 million in federal contracts this year alone.
"It’s shocking how much money is being spent on testing primates," Rep. Nancy Mace, R-S.C., told ABC News.
Mace's district encompasses the Beaufort County community of Yemassee, where the 6,701 primates housed at the sprawling Alpha Genesis facility nearly triple the number of town residents.
In a formal letter to the NIH, the agency that funds laboratory research, and the U.S. Department of Agriculture, which inspects and regulates breeding facilities, Mace expressed "very urgent concerns regarding federal oversight of Alpha Genesis." Mace said the prolonged attempts to recapture all of the primates are "placing the animals and my constituents at risk."
"A lot of constituents were concerned about whether or not the primates that escaped were sick or ill, or have been tested on," Mace told ABC News. "There were a lot of folks concerned about the facility being a breeding facility and the testing that goes on there as well."
The escape highlights an 'issue of national security'
The incident some locals have referred to as "the great escape" has illuminated the international crisis hitting the animal research industry that Alpha Genesis' Westergaard said has become "an issue of national security."
In 2020, the Chinese government, the world's primary breeder of research monkeys, banned the exports of nonhuman primates (NPH) to labs in the United States and elsewhere, triggering an international shortage of the animals just as research scientists were scrambling to come up with vaccines to combat the COVID-19 pandemic, according to a May 2023 report by National Academy of Engineering and National Academy of Medicine.
Primates, according to the NIH-supported report, are valuable in answering certain research questions because of their genetic, anatomic, physiologic and behavioral similarities to humans. However, the China ban on exporting research animals exacerbated the shortage and stalled NIH-funded research, according to the report.
Alpha Genesis federal contracts pre and post-pandemic
The report concluded that the United States "needs to prioritize expansion" of domestic primate breeding programs.
"Relying on importing these animals from other countries is unsustainable, and dependence on international sources undermines the security of the nation’s biomedical research enterprise," the report warned.
In 2021, the National Primate Research Centers could not meet two-thirds of researcher requests for rhesus macaques, according to the report.
"Researchers also face increased wait times for animals, and costs have risen 10% to 200% for a single animal, depending on the species," the report said.
What we know about Alpha Genesis' research
The crisis prompted Alpha Genesis to increase its domestic breeding of research primates. According to Rep. Mace, the company also manages the NIH's so-called "Monkey Island" on Morgan Island in Beaufort County, which holds another 3,300 primates.
Westergaard told ABC News that Alpha Genesis employs 275 people, plus 30 or so contractors.
In addition to breeding lab monkeys, Alpha Genesis provides researchers across the country with biological products and materials, including serum, plasma, whole blood and tissue samples from a wide variety of research species, according to the company's website. The private company's researchers have helped develop several therapeutic drugs and vaccines, including those to treat the COVID-19 virus.
Price of Research Monkeys
Alpha Genesis lab, Adobe stock
According to NIH online records provided to USASpending.gov, the crisis appears to be in accord with a boost in federal contracts Alpha Genesis has received, jumping from $7.3 million in 2021 to $12.3 million in 2022, $14.2 million in 2023 and $19 million this year.
Primates are worth up to $30,000 each
"The price of research monkeys has indeed increased a great deal since the Chinese banned all exports," Westergaard said in an email to ABC News. "Prior to the ban monkeys sold for around $4K - $6K, after the ban prices have increased to $10K - $30K+ due to increased costs of raising animals in the US compared to China. An important point to note is that the shortage remains severe and a great deal of research in the US simply cannot be done because animals are not available at any cost."
Westergaard said some suppliers of laboratory primates have turned to the illegal sourcing of wild-caught monkeys from Cambodia, "which we have not done."
Forty-three rhesus macaque monkey escaped from the Alpha Genesis research lab Castle Hall Road in Yemassee, South Carolina, Nov. 07, 2024.
Yemassee Police Department
"It should also be noted that the Chinese government is seeking worldwide domination in medical research and the development of bio-weapons to target US citizens and our allies," Westergaard said.
He added, "Alpha Genesis is a leading provider of NHPs to the US market and has been instrumental in attempting to fill this void. The alternative is to allow the Chinese to dominate medical development to the severe detriment of our National Security interests."
Human error or intentional act?
Asked by ABC News whether the rapid expansion of Alpha Genesis' breeding and testing operations might have played a role in the escape of the 43 primates, Westergaard said the cause of the escape remains under investigation, including whether it was the result of "human error" or an "intentional act."
"All the information we have thus far indicates that this is human error due to an employee failing to secure containment doors behind her, and a third door directly containing the animals, while doing routine cleaning and feeding," Westergaard said in an email. "The enclosure was brand new and in perfect working order. We continue to investigate in an attempt to determine to the greatest extent possible whether this was or was not an intentional act."
Westergaard said that immediately after the incident occurred, the employee’s supervisor told her she could be fired if it was determined that no structural failure of the primates' enclosure led to the incident. Westergaard said the employee walked off the job and has not returned.
As of early Tuesday, four of the escaped primates remained on the loose, Westergaard said. Two were caught Monday, he said.
"The girls from today are in good health and the others continue to thrive," Westergaard said Monday. "We believe the four monkeys remaining are probably all together either in the area adjacent to our property or somewhere else very close by."
Mace has requested answers from NIH Director Monica Bertagnolli, USDA Deputy Administrator for Animal Care Sarah Helming, and Acting Director Axel Wolf of the NIH's Office of Laboratory Animal Welfare. She also noted that this was not the first time problems had arisen at the research lab.
Mace, who told ABC News she is against animal testing, cited in her letter a September 2022 USDA inspection report of Alpha Genesis that found six separate incidents of animals escaping from their primary enclosures between January and August of 2022. Mace also cited escapes dating back to 2014, when more than two dozen monkeys slipped out of the facility, resulting in a fine from the USDA.
The 2022 USDA inspection report, which ABC News reviewed, also found an infant monkey died after becoming entangled in a stretch of gauze material used in an enclosure to hold a water bottle; said two primates were found dead in their enclosures with their fingers entrapped in structures inside their cages; and documented that one animal died from trauma and four others required veterinary care after they were placed in incorrect enclosures and were attacked by other primates unfamiliar with them.
As a result of the inspection, Alpha Genesis, according to the report, took corrective action to secure enclosures and "made significant changes" to avoid putting primates in the wrong enclosures.
The latest USDA routine inspection of Alpha Genesis lab was conducted on May 21 and concluded, "No non-compliant items identified during this inspection," according to USDA online records.
"This is also true of several other inspections in recent years," Westergaard said. "For a facility of this size that is quite remarkable."
Mace said she met with Westergaard last week to discuss the escape and what Alpha Genesis is doing to round up the monkeys.
“It was an interesting conversation," Mace said. "He tried to tell me how good the primates have it at his facility. And my response was, they have it good until you kill them with disease."
Asked about the conversation, Westergaard responded: "I spoke to the congresswoman last week and at that time she said that she recognized the economic importance of our company to the people of the Low Country and that as a locally-owned business, she would continue to offer her full support."
Alternatives to testing primates
Angela Grimes, CEO of Born Free USA, an international wildlife conservation and animal protection organization, told ABC News that her group has sent a letter to Alpha Genesis offering to rehome the escaped primates to its animal sanctuary in South Texas, where more than 200 rhesus macaque monkeys now reside, including some rescued from U.S. research labs. She said an anonymous donor has pledged $250,000 to help move the animals to the sanctuary.
"What we’d like to see is these animals be released to the Born Free USA sanctuary in South Texas, where they can have some of that freedom that they’ve just gotten a taste of," Grimes said.
Angela Grimes, the CEO of the animal rights group Born Free USA.
Grimes said Alpha Genesis has not responded to her group's offer.
The nonprofit Born Free USA Primate Sanctuary, a 175-acre facility in Dilley, Texas, has been accredited since 2009 by the Global Federation of Animal Sanctuaries, GFAS executive director Valerie Taylor told ABC News, adding that her group conducts rigorous assessments and inspections of animal sanctuaries across the United States and around the globe to ensure the highest standard of animal care possible. Taylor said U.S. animal sanctuaries undergo accreditation every three years and that her organization recently visited the Born Free USA sanctuary as part of the reaccreditation process.
"We meet and exceed GFAS’ highest standards," Grimes said.
Grimes said the medical research industry needs to research alternatives to subjecting primates to experimental testing of deadly diseases.
"I understand human health is important, but I also look at the other viable alternatives that are out there that do not result in the suffering and death of animals," Grimes said.
Westergaard said testing of primates is necessary, though.
"There is no safe or effective way to make the leap from simpler model organisms like mice and rats to humans without using NHPs as an intermediary," Westergaard said. "The therapeutics created using NHPs as research models directly lead to lifesaving and life-prolonging treatments and cures for human disease. Without NHPs as a research model, the world would still be ravaged with wide-spread polio, smallpox wouldn’t be eradicated, and HIV would still be a death sentence."
Sow the wind, reap the whirlwind.
It's gonna be GENERATIONS before people trust medicine again. And government.
Summerthyme
night driver
ESFP adrift in INTJ sea
Yep.
And ALL FULLY EARNED!!!
And ALL FULLY EARNED!!!
Heliobas Disciple
TB Fanatic

Public Health Agency of Canada confirms the first case of clade I mpox in Canada
On November 22, 2024, the Public Health Agency of Canada (PHAC) confirmed the first case of clade I mpox in Canada in an individual in Manitoba.
Public Health Agency of Canada confirms the first case of clade I mpox in Canada
From: Public Health Agency of Canada
Statement
November 22, 2024 | Ottawa, ON | Public Health Agency of Canada
On November 22, 2024, the Public Health Agency of Canada (PHAC) confirmed the first case of clade I mpox in Canada in an individual in Manitoba. This travel-related case is associated with an ongoing outbreak of clade I mpox in central and eastern Africa. The individual sought medical care for mpox symptoms in Canada shortly after their return and is currently isolating. A public health investigation, including contact tracing, is ongoing.
PHAC is working closely with public health authorities in Manitoba. The National Microbiology Laboratory (NML) notified the province on November 22 that the sample tested positive for mpox clade Ib. While clade II mpox has been circulating in Canada since 2022, this is the first case of clade I mpox confirmed in Canada.
The risk to the general population in Canada remains low at this time. PHAC continues to actively monitor the situation and will provide updated information as it becomes available.
Mpox is a viral infection that causes a painful rash. The rash can be accompanied by other symptoms such as fever, chills, headache, exhaustion, swollen lymph nodes, and back, joint and muscle pain. In rare cases, it can be fatal. Mpox is contagious and people in close contact with individuals with mpox, especially those with visible lesions or symptoms, are at higher risk of infection. Children, pregnant people, and those with compromised immune systems, such as those living with HIV or other chronic conditions, are at increased risk of severe disease from clade I mpox.
At this time, vaccination of the general public is not recommended. However, for those at high risk of exposure, getting vaccinated is a key prevention strategy. The Government of Canada has sufficient supply of mpox vaccines to support provincial and territorial programs for the prevention and control of mpox in Canada.
People can also lower their risk of getting mpox by avoiding close physical, including sexual contact, with someone who has mpox or was exposed to mpox; and contact with personal items or objects used by someone who has mpox.
When travelling to countries experiencing outbreaks, individuals should take measures outlined in the travel health notice to reduce their risk, including avoiding contact with someone who has symptoms of mpox or who may have been exposed to mpox.
Individuals who have come into contact with someone who has, or could have, mpox should:
- monitor for symptoms.
- contact their health care provider and local public health unit for information on how to receive post-exposure vaccination as soon as possible. It is preferable to receive the vaccine within 4 days of the last exposure, but it can be offered up to 14 days since the last exposure.
Individuals with symptoms should:
- immediately isolate at home away from others and contact their health care provider.
- if travelling, tell a flight attendant before they land or the border services officer as they enter the country. They will notify a quarantine officer who can assess symptoms and determine further measures.
Early detection, diagnosis, isolation, and contact tracing are key to effectively controlling the transmission of clade I mpox virus in Canada.
psychgirl
TB Fanatic
I saw that this morning too.
Weird.
psychgirl
TB Fanatic
helen
Panic Sex Lady
Urgency now. Both mpox and marburg.
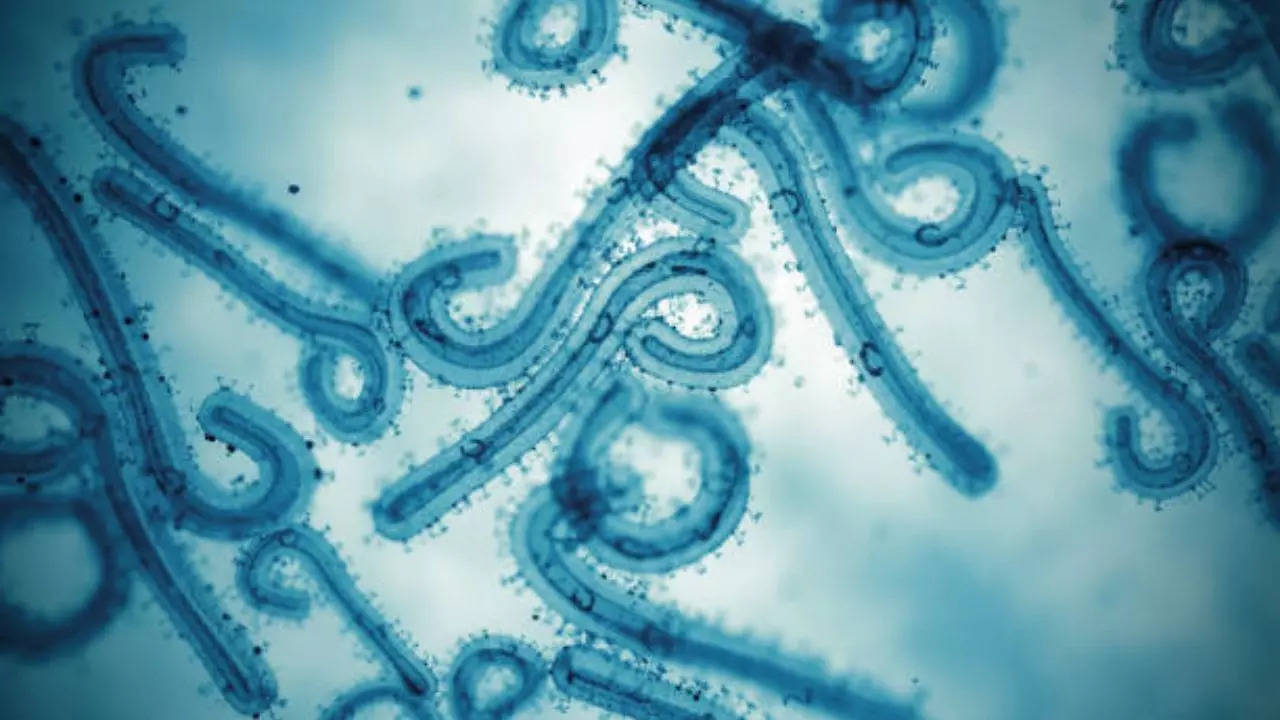
 www.timesnownews.com
www.timesnownews.com

Urgent Warning Issued Over Spread of Deadly Bleeding Eyes Marburg Virus After 15 Die In Africa
Travelers are being advised to stay vigilant due to the spread of Marburg, Mpox, and Oropouche viruses that have spread across Africa, the UK and the Caribbeans. Marburg causes bleeding symptoms, which has killed 15 in Rwanda. Oropouche, spread by midge bites, affects several South American...