You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
CORONA 9/12 Biden to announce more Vaccine Measures (Update Post #189)
- Thread starter phloydius
- Start date
Squib
Veteran Member
Yes
Yes, Countrymouse
Pfizer started testing in March. I did a bunch of research on this recently.
Fauci is hoping the Pfizer vaccine for 5 to 11 will be approved this month. And Moderna in October. They have already been testing 6 month olds.
Oh crap sakes!
Could you point me in the direction of a source?
I’ve got several little grandkids and another on the way!
Squib
Veteran Member
Oh crap sakes!
Could you point me in the direction of a source?
I’ve got several little grandkids and another on the way!
Never mind, I found one article that gives that approximate time line…
Why Is It Taking So Long to Get Vaccines for Kids?
A few things still need to happen before the shots can be authorized for Americans younger than 12.
Updated at 5:10 p.m. ET on August 11, 2021.
The timing of the latest COVID-19 surge isn’t great for children. Millions have already started the school year, the rest will do so in the coming weeks, and COVID-19 vaccines aren’t yet available for the 50 million Americans who haven’t reached their 12th birthday.
Vaccine availability will not bring this pediatric outbreak to a halt. But it will help curb the spread of the virus for everyone, and give many families a better sense of how to plan for the future. To that end, as we hurtle toward the fall, parents, teachers, and pediatricians are eager to know when, exactly, the youngest Americans will have a shot at getting a shot. Even though the timeline is still uncertain, the government and vaccine makers have offered hints to help us understand how the process might unfold.
Vaccines for young kids are most likely to be authorized via the same emergency-use mechanism that allowed adults to get their shots starting last December. The process is a bit of a push-and-pull between vaccine makers and the government. The companies have to recruit participants, perform clinical trials, collect data, and submit that information to the government, and the FDA has to tell the companies what sorts of data it’s looking for, how much, and over what timeline. Once the FDA grants an emergency-use authorization, the CDC has to weigh in, offering recommendations to the nation’s doctors and public-health bodies about when and how the shots should be used. (The latter step took only one day after the FDA authorized each of the Pfizer, Moderna, and Johnson & Johnson vaccines for adults.)
Everyone involved has some control—but not full control—over how long it’s all going to take. A Pfizer spokesperson told me that the company plans to submit an EUA application for the 5-to-11-year-old group “by the end of September,” and for the six-month-to-5-year-old group “shortly thereafter.” Then the FDA will take the reins. After this article was published, a Moderna spokesperson told me that the company’s full data set will likely be ready “later this year or towards the very beginning of 2022,” and that information on different age subgroups might be available at different (as yet unspecified) times.
The FDA has been saying since May that it expects vaccines to be available for kids under 12 on a “fall or winter timeline.” But it hasn’t offered much in the way of updates. When I asked the agency for its best estimate of when it might issue an emergency-use authorization for either the Pfizer or Moderna shots in young kids, a spokesperson referred me to comments that the director of the agency’s Center for Biologics Evaluation and Research made in early July indicating that he expected results from the clinical trials “later this year.”
As we get closer to that amorphous deadline, you can keep an eye out for signs of progress. The first milestone will come when the clinical trials in vaccines for kids stop accepting new participants. Once that happens, the company’s researchers can put all their effort into evaluating the trial itself. (For context, Pfizer finished enrolling 12-to-15-year-olds on January 22 and submitted its application to the FDA on April 9.) As of today, both the Pfizer and Moderna trials are still listed as “recruiting” in the National Library of Medicine’s clinical-trial database; you can check their status here and here, respectively. A more obvious milestone will be reached when either vaccine maker submits an application for an EUA. (Pfizer’s application for 12-to-15-year-olds was approved on May 10, a month after the company submitted it.)
Late last month, The New York Times reported that Pfizer and Moderna were extending the recruitment phases for their clinical trials among young kids at the FDA’s behest, because the agency is concerned about having a large-enough sample size to detect rare side effects. Critics of the agency, including the leadership of the American Academy of Pediatrics, argue that this demand for more participants will make the authorization process drag on for longer than necessary, prolonging the harm caused to kids by not offering them the vaccines.
Exactly how much time the extra recruiting will take isn’t clear. Saad Omer, the director of the Yale Institute for Global Health, told me that several of his friends and colleagues working on the clinical trials have said that parents are eager to sign their kids up, so the new directive might not slow things down very much. The real limiting factor, he said, will be the amount of follow-up that the FDA wants from these new participants. The agency has not made public the exact time period it’s requesting of drugmakers for the pediatric trials. In a document of guidelines for trials in all age groups from June, it recommended at least six months of data on adverse events after each injection before full licensure would be granted—an interval that would push the process back to February, no matter what. Of course, emergency-use authorization could happen sooner—after all, the agency signed off on the shots for adults after only two months’ worth of safety data last year. But there’s no guarantee that it will be similarly lenient with the adverse-effects data for small children.
The current surge in pediatric cases won’t help the trials, either. Rampant spread of the virus last fall did speed up vaccine trials among adults, because it meant that drugmakers had more COVID-19 cases in their data sets, and more evidence to prove that their shots had been effective. But that same grim equation doesn’t hold for the safety data that will be most relevant for kids, because the presence or absence of adverse effects does not depend on people’s being sick.
Couldn’t these questions have been resolved months ago if clinical trials in kids had simply started sooner? As frustrating as the delay is, it’s a common phenomenon in drug testing, which tends to start with healthy adults and then expand to include other populations. Nahid Bhadelia, the director of Boston University’s Center for Emerging Infectious Diseases Policy and Research, told me that people who are immunocompromised, pregnant, or under 18 “tend to get left out in trials,” both because they’re harder to recruit and because of a perception that they’re at higher risk from any potential side effects.
Even if every child in America were made eligible for a vaccine today, they wouldn’t necessarily get one anytime soon. As of two months ago, just over half of parents of 3-to-11-year-olds in one survey said their child would likely not get a shot when it becomes available. And even if every child in America did get a jab today, their immunity wouldn’t ripen until well after Labor Day. Vaccines for the under-12s simply aren’t going to eliminate the anxiety around the back-to-school season.
In the meantime, the strategies we’ve learned to use throughout the pandemic will keep kids safer. Masking, quality ventilation, frequent testing, and vaccinating as many adults and adolescents as possible will all help lower case rates among children. That, in turn, will keep more of them out of the hospital and help them avoid the virus’s still-unknown long-term consequences.
The Atlantic’s COVID-19 coverage is supported by grants from the Chan Zuckerberg Initiative and the Robert Wood Johnson Foundation.
The left is pretty bold. They must feel they have the election good and stolen next year.
They assume that they can handle things inside the hot house while rocks are being thrown at it from the outside.....
BullshitNever mind, I found one article that gives that approximate time line…

Why Is It Taking So Long to Get Vaccines for Kids?
A few things still need to happen before the shots can be authorized for Americans younger than 12.www.theatlantic.com
Updated at 5:10 p.m. ET on August 11, 2021.
The timing of the latest COVID-19 surge isn’t great for children. Millions have already started the school year, the rest will do so in the coming weeks, and COVID-19 vaccines aren’t yet available for the 50 million Americans who haven’t reached their 12th birthday.
Vaccine availability will not bring this pediatric outbreak to a halt. But it will help curb the spread of the virus for everyone, and give many families a better sense of how to plan for the future. To that end, as we hurtle toward the fall, parents, teachers, and pediatricians are eager to know when, exactly, the youngest Americans will have a shot at getting a shot. Even though the timeline is still uncertain, the government and vaccine makers have offered hints to help us understand how the process might unfold.
Vaccines for young kids are most likely to be authorized via the same emergency-use mechanism that allowed adults to get their shots starting last December. The process is a bit of a push-and-pull between vaccine makers and the government. The companies have to recruit participants, perform clinical trials, collect data, and submit that information to the government, and the FDA has to tell the companies what sorts of data it’s looking for, how much, and over what timeline. Once the FDA grants an emergency-use authorization, the CDC has to weigh in, offering recommendations to the nation’s doctors and public-health bodies about when and how the shots should be used. (The latter step took only one day after the FDA authorized each of the Pfizer, Moderna, and Johnson & Johnson vaccines for adults.)
Everyone involved has some control—but not full control—over how long it’s all going to take. A Pfizer spokesperson told me that the company plans to submit an EUA application for the 5-to-11-year-old group “by the end of September,” and for the six-month-to-5-year-old group “shortly thereafter.” Then the FDA will take the reins. After this article was published, a Moderna spokesperson told me that the company’s full data set will likely be ready “later this year or towards the very beginning of 2022,” and that information on different age subgroups might be available at different (as yet unspecified) times.
The FDA has been saying since May that it expects vaccines to be available for kids under 12 on a “fall or winter timeline.” But it hasn’t offered much in the way of updates. When I asked the agency for its best estimate of when it might issue an emergency-use authorization for either the Pfizer or Moderna shots in young kids, a spokesperson referred me to comments that the director of the agency’s Center for Biologics Evaluation and Research made in early July indicating that he expected results from the clinical trials “later this year.”
As we get closer to that amorphous deadline, you can keep an eye out for signs of progress. The first milestone will come when the clinical trials in vaccines for kids stop accepting new participants. Once that happens, the company’s researchers can put all their effort into evaluating the trial itself. (For context, Pfizer finished enrolling 12-to-15-year-olds on January 22 and submitted its application to the FDA on April 9.) As of today, both the Pfizer and Moderna trials are still listed as “recruiting” in the National Library of Medicine’s clinical-trial database; you can check their status here and here, respectively. A more obvious milestone will be reached when either vaccine maker submits an application for an EUA. (Pfizer’s application for 12-to-15-year-olds was approved on May 10, a month after the company submitted it.)
Late last month, The New York Times reported that Pfizer and Moderna were extending the recruitment phases for their clinical trials among young kids at the FDA’s behest, because the agency is concerned about having a large-enough sample size to detect rare side effects. Critics of the agency, including the leadership of the American Academy of Pediatrics, argue that this demand for more participants will make the authorization process drag on for longer than necessary, prolonging the harm caused to kids by not offering them the vaccines.
Exactly how much time the extra recruiting will take isn’t clear. Saad Omer, the director of the Yale Institute for Global Health, told me that several of his friends and colleagues working on the clinical trials have said that parents are eager to sign their kids up, so the new directive might not slow things down very much. The real limiting factor, he said, will be the amount of follow-up that the FDA wants from these new participants. The agency has not made public the exact time period it’s requesting of drugmakers for the pediatric trials. In a document of guidelines for trials in all age groups from June, it recommended at least six months of data on adverse events after each injection before full licensure would be granted—an interval that would push the process back to February, no matter what. Of course, emergency-use authorization could happen sooner—after all, the agency signed off on the shots for adults after only two months’ worth of safety data last year. But there’s no guarantee that it will be similarly lenient with the adverse-effects data for small children.
The current surge in pediatric cases won’t help the trials, either. Rampant spread of the virus last fall did speed up vaccine trials among adults, because it meant that drugmakers had more COVID-19 cases in their data sets, and more evidence to prove that their shots had been effective. But that same grim equation doesn’t hold for the safety data that will be most relevant for kids, because the presence or absence of adverse effects does not depend on people’s being sick.
Couldn’t these questions have been resolved months ago if clinical trials in kids had simply started sooner? As frustrating as the delay is, it’s a common phenomenon in drug testing, which tends to start with healthy adults and then expand to include other populations. Nahid Bhadelia, the director of Boston University’s Center for Emerging Infectious Diseases Policy and Research, told me that people who are immunocompromised, pregnant, or under 18 “tend to get left out in trials,” both because they’re harder to recruit and because of a perception that they’re at higher risk from any potential side effects.
Even if every child in America were made eligible for a vaccine today, they wouldn’t necessarily get one anytime soon. As of two months ago, just over half of parents of 3-to-11-year-olds in one survey said their child would likely not get a shot when it becomes available. And even if every child in America did get a jab today, their immunity wouldn’t ripen until well after Labor Day. Vaccines for the under-12s simply aren’t going to eliminate the anxiety around the back-to-school season.
In the meantime, the strategies we’ve learned to use throughout the pandemic will keep kids safer. Masking, quality ventilation, frequent testing, and vaccinating as many adults and adolescents as possible will all help lower case rates among children. That, in turn, will keep more of them out of the hospital and help them avoid the virus’s still-unknown long-term consequences.
The Atlantic’s COVID-19 coverage is supported by grants from the Chan Zuckerberg Initiative and the Robert Wood Johnson Foundation.
Chance
Veteran Member
Reuters has an article titled 'US FDA may approve covid vaccine after two months of safety testing' from a couple of days ago...or similar title...I'd link it but I'm on my phone.....the article says 6 months to 2 years old could be approved October, November. Pfizer is hurrying all of this under emergency use authorization. Reading October for 6 to 11 years olds in this same article. Not good for our kids.Oh crap sakes!
Could you point me in the direction of a source?
I’ve got several little grandkids and another on the way!
Not good at all.Reuters has an article titled 'US FDA may approve covid vaccine after two months of safety testing' from a couple of days ago...or similar title...I'd link it but I'm on my phone.....the article says 6 months to 2 years old could be approved October, November. Pfizer is hurrying all of this under emergency use authorization. Reading October for 6 to 11 years olds in this same article. Not good for our kids.
Fastest way I know to die is f****** with someone's kids.
Raggedyman
Res ipsa loquitur
Not good at all.
Fastest way I know to die is f****** with someone's kids.
BOOM!!!!
Not good at all.
Fastest way I know to die is f****** with someone's kids.
and guns.
heelgeneral
Senior Member
Do the Amish take converts?
I've been having the same thought a lot lately.
Countrymouse
Country exile in the city
Oh crap sakes!
Could you point me in the direction of a source?
I’ve got several little grandkids and another on the way!
Squib--I guess you saw this post that Xtreme Right put up--?
It was originally posted by DazedandConfused in reply to Tammy in WI, and he KNEW the child and her parents.......
Of course, that's all "ANECDOTAL", so to some this little girl's death means nothing if some doctor didn't put her in a STUDY ....
Tristan
Has No Life - Lives on TB
He can sign stuff but can he enforce it?
He has minions for that. So the question is, do the minions continue minoning?
Tristan
Has No Life - Lives on TB
The left is pretty bold. They must feel they have the election good and stolen next year.
Gee, I wonder why they would think that?
The dominions willHe has minions for that. So the question is, do the minions continue minoning?
Ragnarok
On and On, South of Heaven
He has minions for that. So the question is, do the minions continue minoning?

Last time it took 75 million deaths ( approximately ).
World War II was the deadliest military conflict in history in terms of total dead, with some 75 million people casualties including military and civilians, or around 3% of the world's population at the time
3 percent of the current population of 7.9 billion is 237,000,000...
How many die during the Tribulation according to the Bible?
...
... The minions will continue to "minioning" up until the very end.
phloydius
Veteran Member
As posted by jed turtle on another thread:
“The next session for the U.N. General Assembly starts Tuesday, Sept. 14, in New York City, and the first day of general debate begins the following week. It’s not clear why Murthy tied new COVID-19 measures with the U.N. meeting.”
 nworeport.me
nworeport.me
Biden to Announce Even More COVID-19 Measures This Week: Surgeon General
Date: September 13, 2021Author: Nwo Report0 Comments

Source: Jack Phillips
President Joe Biden will announce more COVID-19 measures before the United Nations General Assembly meets this week, said U.S. Surgeon General Vivek Murthy.
Speaking to CNN on Sunday, Murthy did not say what new measures would be announced by Biden.
Last week, the president issued several executive orders mandating that federal contractors, federal workers, and most healthcare staff in the United States get the COVID-19 vaccine. A White House plan also said he would direct the Occupational Safety and Health Administration, or OSHA, to mandate that employers with 100 or more workers either get the vaccine, require weekly COVID-19 testing, or get fined.
Biden’s announcement on the mandate, which will affect 80 million estimated private-sector employees, drew significant criticism from Republican governors and some business leaders. It’s likely that numerous lawsuits will be filed against the order.
But during the CNN interview, Murthy defended the president’s announcement and mandate to attempt to increase vaccination.
“There will be more actions that we continue to work on, especially in the global front,” he said.China in Focus
The next session for the U.N. General Assembly starts Tuesday, Sept. 14, in New York City, and the first day of general debate begins the following week. It’s not clear why Murthy tied new COVID-19 measures with the U.N. meeting.
“What the president and what all of us have said as public health leaders from the earliest part of this pandemic is that we have to use every level of government, and we all in the private sector have to do everything we can to tackle this virus,” Murthy also said. “The requirements the president announced are an example of that.”
In a separate interview with ABC News, Murthy also defended the mandates, saying that when private companies such as Tyson Foods made vaccines a requirement, it increased the U.S. vaccination rate.
“We know that these kinds of requirements actually work to improve our vaccination rates,” Murthy said. “Tyson Foods, for example, which put in a vaccine requirement recently saw that its vaccination rate went from 45 percent to more than 70 percent in a very short period of time and they’re not even at their deadline yet.”
But Republicans said they will file lawsuits against the mandate when it is handed down by the federal government. They described the forthcoming rules as draconian and designed to further divide Americans.
“I’ve been talking to my attorney general, he is coordinating with the other attorneys general across the country who share similar views about the overreach,” Nebraska Gov. Pete Ricketts told Fox News on Sunday.
“As we see what these rules are we will be able to know exactly how we will be able to challenge them in court. I’m also talking with my colleagues around the country as well the other governors who feel the way I do, and we’ll be working on other strategies,” he added.
Arkansas Gov. Asa Hutchinson told CNN Sunday that the vaccine mandates will “[harden] the resistance” that some individuals have to vaccines.
“I’m trying to overcome [vaccine] resistance, but the president’s actions in a mandate hardens the resistance,” he said.
Other governors suggested they would follow suit.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden administration,” Georgia Gov. Brian Kemp wrote last week. Texas Gov. Greg Abbott, meanwhile, called the rule an “assault on private businesses,” adding that “Texas is already working to halt this power grab.”
The Epoch Times has contacted the White House for additional comment.
“The next session for the U.N. General Assembly starts Tuesday, Sept. 14, in New York City, and the first day of general debate begins the following week. It’s not clear why Murthy tied new COVID-19 measures with the U.N. meeting.”
Biden to Announce Even More COVID-19 Measures This Week: Surgeon General
Source: Jack Phillips President Joe Biden will announce more COVID-19 measures before the United Nations General Assembly meets this week, said U.S. Surgeon General Vivek Murthy. Speaking to CNN on Sunday, Murthy did not say what new measures would be announced by Biden. Last week, the president...
 nworeport.me
nworeport.me
Biden to Announce Even More COVID-19 Measures This Week: Surgeon General
Date: September 13, 2021Author: Nwo Report0 Comments

Source: Jack Phillips
President Joe Biden will announce more COVID-19 measures before the United Nations General Assembly meets this week, said U.S. Surgeon General Vivek Murthy.
Speaking to CNN on Sunday, Murthy did not say what new measures would be announced by Biden.
Last week, the president issued several executive orders mandating that federal contractors, federal workers, and most healthcare staff in the United States get the COVID-19 vaccine. A White House plan also said he would direct the Occupational Safety and Health Administration, or OSHA, to mandate that employers with 100 or more workers either get the vaccine, require weekly COVID-19 testing, or get fined.
Biden’s announcement on the mandate, which will affect 80 million estimated private-sector employees, drew significant criticism from Republican governors and some business leaders. It’s likely that numerous lawsuits will be filed against the order.
But during the CNN interview, Murthy defended the president’s announcement and mandate to attempt to increase vaccination.
“There will be more actions that we continue to work on, especially in the global front,” he said.China in Focus
The next session for the U.N. General Assembly starts Tuesday, Sept. 14, in New York City, and the first day of general debate begins the following week. It’s not clear why Murthy tied new COVID-19 measures with the U.N. meeting.
“What the president and what all of us have said as public health leaders from the earliest part of this pandemic is that we have to use every level of government, and we all in the private sector have to do everything we can to tackle this virus,” Murthy also said. “The requirements the president announced are an example of that.”
In a separate interview with ABC News, Murthy also defended the mandates, saying that when private companies such as Tyson Foods made vaccines a requirement, it increased the U.S. vaccination rate.
“We know that these kinds of requirements actually work to improve our vaccination rates,” Murthy said. “Tyson Foods, for example, which put in a vaccine requirement recently saw that its vaccination rate went from 45 percent to more than 70 percent in a very short period of time and they’re not even at their deadline yet.”
But Republicans said they will file lawsuits against the mandate when it is handed down by the federal government. They described the forthcoming rules as draconian and designed to further divide Americans.
“I’ve been talking to my attorney general, he is coordinating with the other attorneys general across the country who share similar views about the overreach,” Nebraska Gov. Pete Ricketts told Fox News on Sunday.
“As we see what these rules are we will be able to know exactly how we will be able to challenge them in court. I’m also talking with my colleagues around the country as well the other governors who feel the way I do, and we’ll be working on other strategies,” he added.
Arkansas Gov. Asa Hutchinson told CNN Sunday that the vaccine mandates will “[harden] the resistance” that some individuals have to vaccines.
“I’m trying to overcome [vaccine] resistance, but the president’s actions in a mandate hardens the resistance,” he said.
Other governors suggested they would follow suit.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden administration,” Georgia Gov. Brian Kemp wrote last week. Texas Gov. Greg Abbott, meanwhile, called the rule an “assault on private businesses,” adding that “Texas is already working to halt this power grab.”
The Epoch Times has contacted the White House for additional comment.
phloydius
Veteran Member
Maybe related...
From link: "Dr. Fauci calls for unvaccinated Americans to be banned from air travel and to mandate #COVID19 vaccines for school children."
View: https://twitter.com/disclosetv/status/1437382113548980232
From link: "Dr. Fauci calls for unvaccinated Americans to be banned from air travel and to mandate #COVID19 vaccines for school children."
View: https://twitter.com/disclosetv/status/1437382113548980232
bw
Fringe Ranger
"Dr. Fauci calls for unvaccinated Americans to be banned from air travel and to mandate #COVID19 vaccines for school children."
You know, I'm starting to dislike that guy.
TxGal
Day by day
Do the Amish take converts?
I laughed out loud at that, but then wondered enough to look it up!
Some communities will, if they are open to outsiders....some are more closed groups. You'd have to learn old German, give up all your possessions, and of course take to their ways. Apparently that is the hardest for converts, adjusting to the plain life.
There are actually several articles out there on that subject.
Squib
Veteran Member
Squib--I guess you saw this post that Xtreme Right put up--?
It was originally posted by DazedandConfused in reply to Tammy in WI, and he KNEW the child and her parents.......
Of course, that's all "ANECDOTAL", so to some this little girl's death means nothing if some doctor didn't put her in a STUDY ....
Yes, I did, I just couldn’t find the OP for that so Icould go to a source article…I wanted to be able to send the article to others as I said, I have several grandchildren and another on the way…so this is bull crap!
EMICT
Veteran Member
Panel Of Scientists Determines Most People Don't Need Boosters; Moderna Plummets
MONDAY, SEP 13, 2021 - 10:17 AM
As a growing chorus of scientists and activists question the Biden Administration's push to roll out booster jabs to Americans as quickly as possible, more research has just been released suggesting booster jabs simply aren't necessary for most people - especially those who have already been vaccinated once.
The finding comes via a report citing the opinions of an all-star panel of scientists from around the world published via the Lancet. Governments would be better served to focus on immunizing the unvaccinated and to wait for more data on which boosters. What's more, it's not yet clear what doses would be most effective according to the authors, a group that includes two prominent US FDA
experts.
Shares of Moderna, Pfizer and BioNTech traded lower on the news given that boosters were their key to an annual "cash cow" business.
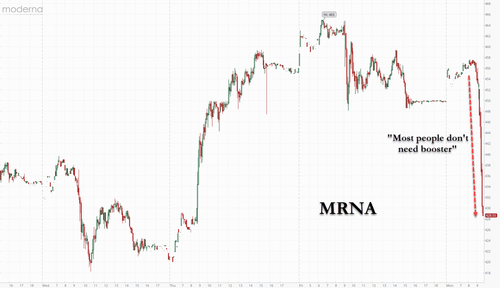
The scientists' assessment was based on a wide range of real-world observational studies as well as data from clinical trials before the vaccines were approved. "None of the studies has provided credible evidence of substantially declining protection against severe disease," the authors wrote.
The FDA scientists involved were Marion Gruber, who leads the FDA’s Office of Vaccines Research and Review, and her deputy Philip Krause. Both recently announced their plans to step down later this year. Now it's pretty clear that they're perhaps doing so to oppose the booster jab rollout.
The WHO has urged against broad use of boosters, saying it would make better public-health sense to focus on immunizing those who haven’t gotten any shots yet whether because of anti-vaccine sentiment in countries with ample reserves, or because they live in places with little access to shots.
"Even if boosting were eventually shown to decrease the medium-term risk of serious disease, current vaccine supplies could save more lives if used in previously unvaccinated populations," the authors wrote.
What's more, boosters could lead to even more harmful side effects in the population, a phenomenon which isn't well understood, even as cases of heart inflammation have increasingly been tied to mRNA vaccines.
MONDAY, SEP 13, 2021 - 10:17 AM
As a growing chorus of scientists and activists question the Biden Administration's push to roll out booster jabs to Americans as quickly as possible, more research has just been released suggesting booster jabs simply aren't necessary for most people - especially those who have already been vaccinated once.
The finding comes via a report citing the opinions of an all-star panel of scientists from around the world published via the Lancet. Governments would be better served to focus on immunizing the unvaccinated and to wait for more data on which boosters. What's more, it's not yet clear what doses would be most effective according to the authors, a group that includes two prominent US FDA
experts.
Shares of Moderna, Pfizer and BioNTech traded lower on the news given that boosters were their key to an annual "cash cow" business.

The scientists' assessment was based on a wide range of real-world observational studies as well as data from clinical trials before the vaccines were approved. "None of the studies has provided credible evidence of substantially declining protection against severe disease," the authors wrote.
The FDA scientists involved were Marion Gruber, who leads the FDA’s Office of Vaccines Research and Review, and her deputy Philip Krause. Both recently announced their plans to step down later this year. Now it's pretty clear that they're perhaps doing so to oppose the booster jab rollout.
The WHO has urged against broad use of boosters, saying it would make better public-health sense to focus on immunizing those who haven’t gotten any shots yet whether because of anti-vaccine sentiment in countries with ample reserves, or because they live in places with little access to shots.
"Even if boosting were eventually shown to decrease the medium-term risk of serious disease, current vaccine supplies could save more lives if used in previously unvaccinated populations," the authors wrote.
What's more, boosters could lead to even more harmful side effects in the population, a phenomenon which isn't well understood, even as cases of heart inflammation have increasingly been tied to mRNA vaccines.
von Koehler
Has No Life - Lives on TB
I read a series of papers on just that very subject.
They basically called it an army of one.
Now take that army of 1 and multiply it times 1 million
There would be no way to stop it.
In my mind I remember the stand-off at a ranch between the .fedgov and a rancher (Brady?). The feds were everywhere around the house but at a nearby overpass were many armed men aimed at the feds.
The standoff lasted for days until the fed's backed down.
The feds knew it wouldn't go well for him.
.fedgov strength is at its best when overwhelming an individual but fades away against determined opposition.
Last edited:
Spot onIn my mind I remember the stand-off at a ranch between the .fedgov and a rancher. The feds were everywhere around the house but at a nearby overpass were many armed men aimed at the feds.
The standoff lasted for days until the fed's backed down.
The feds knew it wouldn't go well for him.
.fedgov strength is at its best when overwhelming an individual.but fades away against determined opposition.
Henry Bowman
Veteran Member
However because nothing ended up happening there, many were picked up, 1 by 1 because they allowed themselves to be photographed . Most, just like the Patriots from Jan. 6th , had no charges filed against them, they were railroaded anyway.In my mind I remember the stand-off at a ranch between the .fedgov and a rancher. The feds were everywhere around the house but at a nearby overpass were many armed men aimed at the feds.
The standoff lasted for days until the fed's backed down.
The feds knew it wouldn't go well for him.
.fedgov strength is at its best when overwhelming an individual.but fades away against determined opposition.
There is a moral to that story.
von Koehler
Has No Life - Lives on TB
Fauci said earlier this summer he expected 6 month olds vaccinated the start of 2022. He'll probably make sure that happens.
Going after children and babies! This is an abhorrent thing to do. They have no voice. It is up to us. - adult citizens, parents, grandparents to speak up and stop this. They should never have been allowed to 'vaccinate' with this in the first place.
Their evil knows no bounds.
von Koehler
Has No Life - Lives on TB
However because nothing ended up happening there, many were picked up, 1 by 1 because they allowed themselves to be photographed . Most, just like the Patriots from Jan. 6th , had no charges filed against them, they were railroaded anyway.
There is a moral to that story.
So true.
PghPanther
Has No Life - Lives on TB
I admit I'm a bit afraid of what he's going to do to us.
"When governments fear the people, there is liberty. When the people fear the government, there is tyranny. The strongest reason for the people to retain the right to keep and bear arms is, as a last resort, to protect themselves against tyranny in government." - Thomas Jefferson
"When governments fear the people, there is liberty. When the people fear the government, there is tyranny. The strongest reason for the people to retain the right to keep and bear arms is, as a last resort, to protect themselves against tyranny in government." - Thomas Jefferson
Displaced hillbilly
Veteran Member
Me too.I've been having the same thought a lot lately.
LittleYellowFlower
Flower Whisperer
Let me know when you find out!!Do the Amish take converts?
Older people more likely to experience severe breakthrough COVID-19, study finds
Erica Carbajal - Thursday, September 9th, 2021 Print | Email
The median age of fully vaccinated people who developed a severe breakthrough COVID-19 infection from March through June was 80, a study published Sept. 7 in The Lancet Infectious Diseases found.
Erica Carbajal - Thursday, September 9th, 2021 Print | Email
The median age of fully vaccinated people who developed a severe breakthrough COVID-19 infection from March through June was 80, a study published Sept. 7 in The Lancet Infectious Diseases found.
phloydius
Veteran Member
Older people more likely to experience severe breakthrough COVID-19, study finds
Erica Carbajal - Thursday, September 9th, 2021 Print | Email
The median age of fully vaccinated people who developed a severe breakthrough COVID-19 infection from March through June was 80, a study published Sept. 7 in The Lancet Infectious Diseases found.
Do you have a link to the study? I'd like to look at it.
The median age of fully vaccinated people who developed a severe breakthrough COVID-19 infection from March through June was 80, a study published Sept. 7 in The Lancet Infectious Diseases found.Do you have a link to the study? I'd like to look at it.
The research was based on an analysis of 969 COVID-19 patients who were admitted to Yale New Haven (Conn.) Health between March 23 and July 1, 2021. Of those, 103 had received at least one dose of a COVID-19 vaccine, and 54 were considered fully vaccinated.
Researchers considered the 54 fully vaccinated people who contracted COVID-19 as breakthrough cases, meaning they began experiencing symptoms or received a positive test at least 14 days after their final dose. Twenty-five (46 percent) of these patients were admitted to the hospital for reasons unrelated to COVID-19 and received an incidental positive test, meaning they were asymptomatic.
A total of 14 breakthrough patients (26 percent) had severe or critical illness. The median age of these patients was 80.5 years. Most of these patients had heart disease, seven had lung disease, and seven had diabetes. All 14 of the patients with severe or critical illness were on ventilators, four were admitted to the intensive care unit and three died, researchers said.
"Identifying who is more likely to develop severe COVID-19 illness after vaccination will be critical to ongoing efforts to mitigate the impact of these breakthrough infections," said Hyung Chun, MD, associate professor of cardiology at Yale School of Medicine. "These cases are extremely rare, but they are becoming more frequent as variants emerge and more time passes since patients are vaccinated."
phloydius
Veteran Member
The median age of fully vaccinated people who developed a severe breakthrough COVID-19 infection from March through June was 80, a study published Sept. 7 in The Lancet Infectious Diseases found.
...
Interesting! Do you have the link to the study so I can read the study?
mzkitty
I give up.
Nasty vaxxers:

Democracy_420
@democracy_16
·
5m
It would a good way to handle the antivax ****ers ****ing up everything like their miserable lives encircling sadness so pathetic #therapy #BreakingNews #BREAKING #boston #parent #vaccine #Jan6 #SeditionHunters #monday
Quote Tweet

SassyGirl914
@ToniC8
· 35m
I think Biden should tell all the antivaxxers they aren't allowed a shot anymore, and that they missed the deadline. They're now locked out of everything. They'd storm the hospitals looking for vaccines.

Democracy_420
@democracy_16
·
5m
It would a good way to handle the antivax ****ers ****ing up everything like their miserable lives encircling sadness so pathetic #therapy #BreakingNews #BREAKING #boston #parent #vaccine #Jan6 #SeditionHunters #monday
Quote Tweet

SassyGirl914
@ToniC8
· 35m
I think Biden should tell all the antivaxxers they aren't allowed a shot anymore, and that they missed the deadline. They're now locked out of everything. They'd storm the hospitals looking for vaccines.
phloydius
Veteran Member

Surgeon General Just Issued This "Curveball" Warning
With health experts adamant that the only way to stop the coronavirus pandemic is to get more people vaccinated, President Joe Biden announced a six point plan last week to do just that, including vaccine mandates for many American workers. How does this affect you? Dr. Vivek Murthy, the...
From the link:
Dr. Vivek Murthy, the nation's Surgeon General, appeared on CNN's State of the Union...
Bash asked Murthy if he feared people would claim religious exemption to get out of taking the vaccine. "That's always a possibility," said Murthy. "It's something that we have fortunately had experience with as a country. We've had vaccine requirements for a number of other illnesses. We do that in schools, for example, you and I went to grade school, we likely had to make sure and confirm that we had certain vaccines in place before we actually came to school. So fortunately, as a country, we have experienced in dealing with exemptions, but we've got to be vigilant there and make sure that people are using them in the spirit that they're intended, and not abusing them or asking for exemptions when they don't apply. That's the main area that we continue to monitor in the days and weeks ahead."
{Emphasis Mine)

