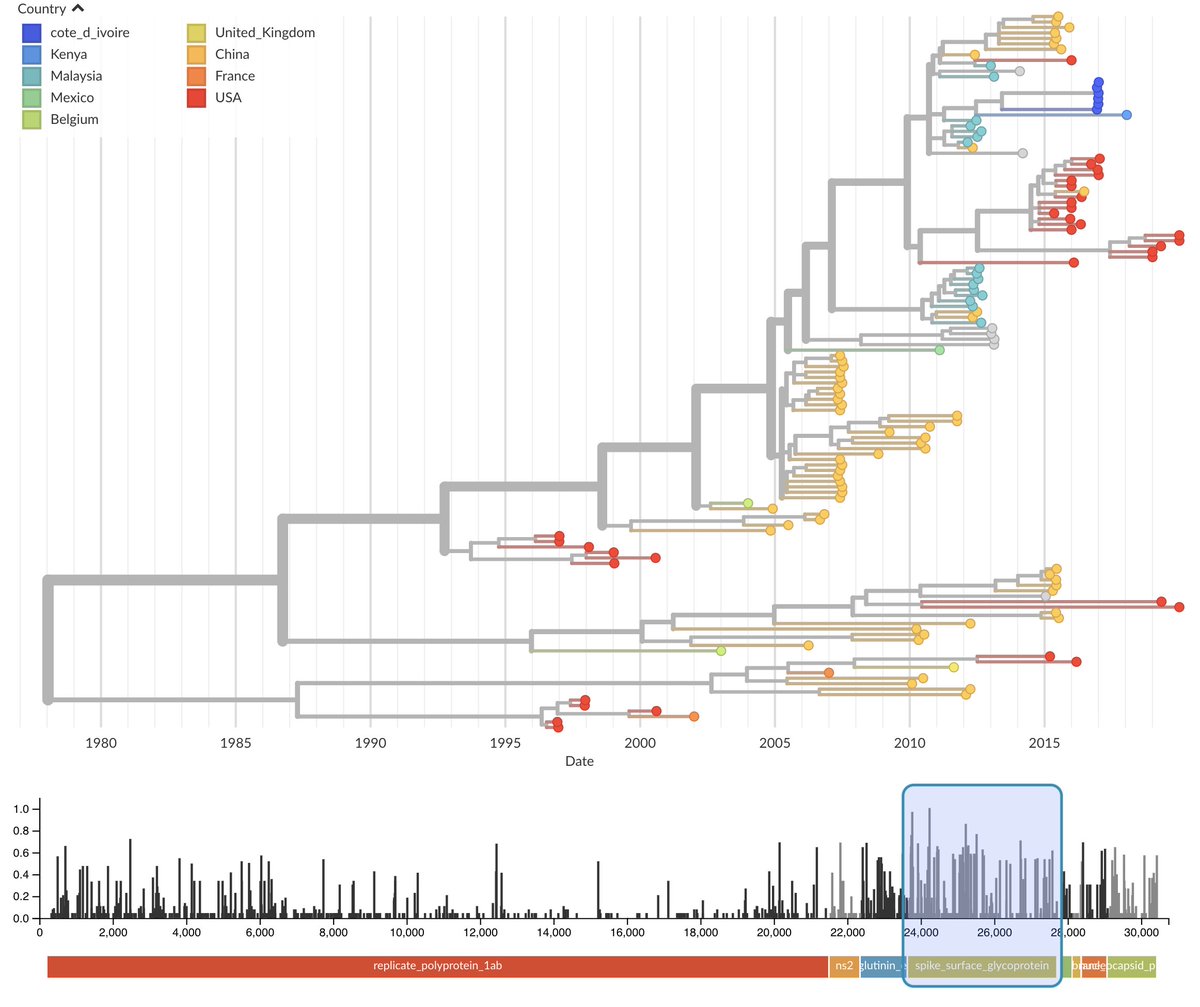
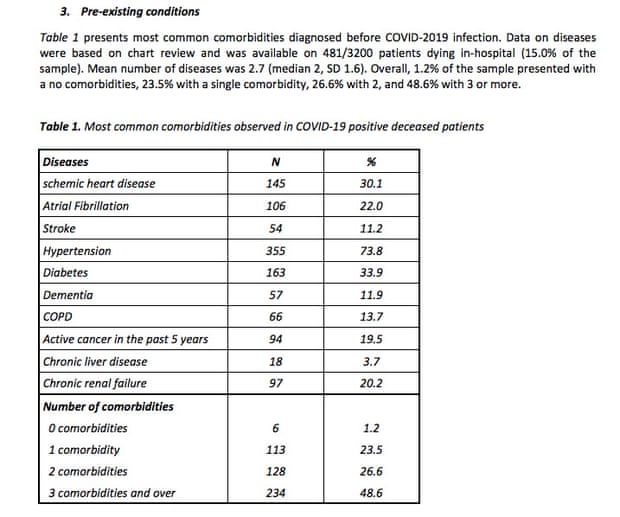
As California fights the coronavirus, there's another danger that's fast approaching towards the state: a nursing and medical staffing shortage could cripple the healthcare system just when we need it most.

sacramento.cbslocal.com
Fighting Coronavirus: Medical Students Near Graduation Pushing To Get On Frontlines
by Rick BooneMarch 23, 2020 at 10:43 pm
CALIFORNIA (CBS13) — As California fights the coronavirus, there’s another danger that’s fast approaching towards the state: a nursing and medical staffing shortage that could cripple the healthcare system just when we need it most.
Medical students near graduation day are pushing Governor Newsom to change the rules so they can get to the frontlines right now.
As many as 14,000 graduating nurses are waiting on the sidelines, pleading with the governor for their early certification now, versus next month.
“I’m so ready, I’ve been preparing for 19 months, I don’t want to do anything else with my life,” nursing student Ryane Panasewicz said.
Panasewicz, who’s just a few weeks from graduating as a nurse, is on a mission to change California’s medical requirements and save lives in this pandemic.
READ:
Hospital Taking Donations Amid Nationwide Supply Shortage
She and a fellow student lobbied that change to lawmakers with an online petition, and so far she’s gotten 90,000 signatures.
“Because we want to graduate and get out there. So this is our way to kinda push back,” she said.
Right now, for a nurse to graduate in the state, they must have hours of on the ground hospital training, known as clinical rotations. But with the coronavirus surging, those rotations have been suspended while career medical professionals focus on the influx of COVID-19 patients.
Some hospital employees agree with the students. Some nurses were protesting Monday to highlight the fact that they’re running thin on everything from equipment to people.
“We’re afraid that too many people are going to start getting sick and there won’t be anyone to take care of the patients anymore,” registered nurse Diane McClure said.
Across the country, several states have waived clinical rotations for graduating nursing students, while others are considering the decision. On Monday, Governor Newsom said he’s now warming up to the idea.
“We believe the ability to get fourth-year medical students into the system… getting someone that’s almost finished getting their nursing degree, get them licensed earlier,” Newsom said.
If he rescinds the requirement, some 14,000 new medical professionals could flow into hospitals immediately. It’s a change students say could have a healing impact on us all.
“I’m very optimistic he’s going to make some changes this week,” Panasewicz said.
There’s also a push to call up retired and reserve doctors and nurses to help battle the virus.













 I'm a "He" and my Ex is a "She".
I'm a "He" and my Ex is a "She".