You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
VIDEO Ivermectin and Colon Cancer
- Thread starter Hacker
- Start date
Hacker
Computer Hacking Pirate
In this video, we're talking about new research into the potential effect of Ivermectin on Colon Cancer cells.
Link to referenced study: Ivermectin has New Application in Inhibiting Colorectal Cancer Cell Growth
Colorectal cancer (CRC) is the third most common cancer worldwide and still lacks effective therapy. Ivermectin, an antiparasitic drug, has been shown to possess anti-inflammation, anti-virus, and antitumor properties. However, whether ivermectin affects CRC is still unclear. The objective of this study was to evaluate the influence of ivermectin on CRC using CRC cell lines SW480 and SW1116. We used CCK-8 assay to determine the cell viability, used an optical microscope to measure cell morphology, used Annexin V-FITC/7-AAD kit to determine cell apoptosis, used Caspase 3/7 Activity Apoptosis Assay Kit to evaluate Caspase 3/7 activity, used Western blot to determine apoptosis-associated protein expression, and used flow cytometry and fluorescence microscope to determine the reactive oxygen species (ROS) levels and cell cycle. The results demonstrated that ivermectin dose-dependently inhibited colorectal cancer SW480 and SW1116 cell growth, followed by promoting cell apoptosis and increasing Caspase-3/7 activity. Besides, ivermectin upregulated the expression of proapoptotic proteins Bax and cleaved PARP and downregulated antiapoptotic protein Bcl-2. Mechanism analysis showed that ivermectin promoted both total and mitochondrial ROS production in a dose-dependent manner, which could be eliminated by administering N-acetyl-l-cysteine (NAC) in CRC cells. Following NAC treatment, the inhibition of cell growth induced by ivermectin was reversed. Finally, ivermectin at low doses (2.5 and 5 µM) induced CRC cell arrest. Overall, ivermectin suppressed cell proliferation by promoting ROS-mediated mitochondrial apoptosis pathway and inducing S phase arrest in CRC cells, suggesting that ivermectin might be a new potential anticancer drug therapy for human colorectal cancer and other cancers.
Ivermectin is a derivative of the 16-membered macrolide compound abamectin, which was first widely used in clinical practice as an antiparasitic drug (Laing et al., 2017). Ivermectin can increase the activity of γ-aminobutyric acid receptor or glutamate-chloride ion channel (Glu-Cl), increase the influx of chloride ions, and cause the cell membrane hyperpolarization, thereby blocking signal transmission between neurons and muscles (Martin et al., 2021), which exerts its antiparasitic effects. Ivermectin could be used, in addition to as an antiparasitic drug, as antiviral agents such as Flavivirus, HIV-1 virus, and SARS-CoV-2 virus (Mastrangelo et al., 2012; Wagstaff et al., 2012; Caly et al., 2020). Moreover, studies have shown that ivermectin has an inhibitory effect on various tumor cells and may be a potential broad-spectrum antitumor drug (Juarez et al., 2020). Juarez et al. (2020) have demonstrated that ivermectin is the most sensitive to breast cancer cells MDA-MB-231, MDA-MB-468, MCF-7, and ovarian SKOV-3; whereas ivermectin is the most nonsensitive to the prostate cancer cell line DU145. The induction of cell cycle arrest at G0/G1 mediates this effect of ivermectin on these sensitive cancer cells. Furthermore, ivermectin can inhibit the proliferation of cancer cells through p21-activated kinase 1 (PAK1)-induced autophagy, Caspase-dependent apoptosis, or immunogenic cell death regulate the signal pathways, including Hippo, Akt/mTOR, and WNT-TCF pathways to inhibit cancer cell proliferation (Liu et al., 2020). As known, ROS plays a vital role in the apoptosis caused by oxidative stress. ROS is a by-product of normal mitochondrial respiration. Stimuli such as infection, drought, cold, and ultraviolet light result in increased ROS in cells. Then, accumulative ROS could induce cells mitochondrial dysfunction and promote apoptosis in cells (Sinha et al., 2013). Evidence has shown that ivermectin-induced apoptosis is closely related to the production of ROS. Currently, there are few reports on the research of ivermectin in colorectal cancer.
Furthermore, new use of old drugs (that is, drug relocation) is a strategy for expanding old drugs and developing new uses, which has the advantages of low research and development cost and short development time (Pushpakom et al., 2019). Research on drug relocation of ivermectin is a shortcut to developing new antitumor drugs. Given this, we designed a study to explore the impact of ivermectin on the proliferation and apoptosis of CRC cells and the underlying mechanism.
FIGURE 1
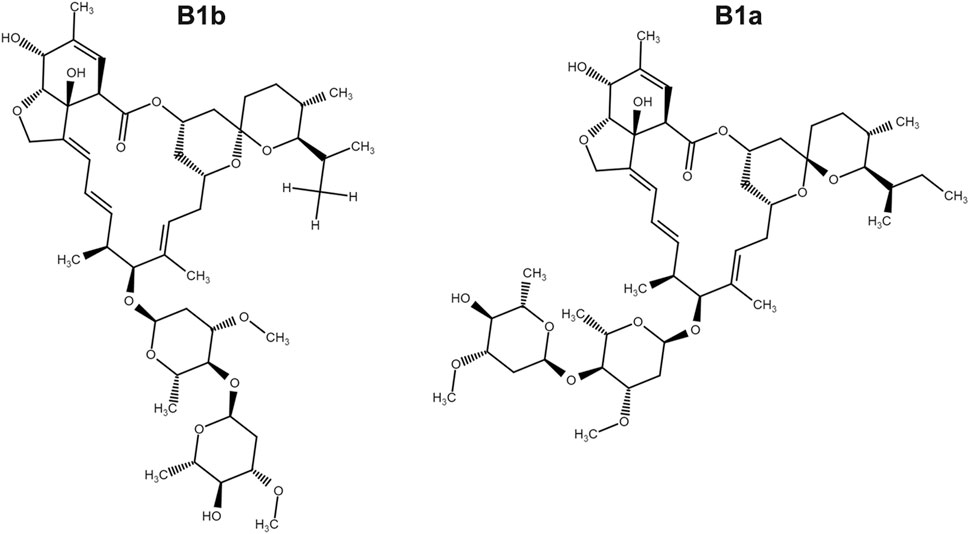
FIGURE 1. The chemical structure of ivermectin which is composed of ivermectin B1a (>90%) and ivermectin B1b.
For the mitochondrial ROS measurement, colorectal cancer cells (SW1116) were seeded in a 12-well plate and incubated overnight. After that, cells were treated with 0, 2.5, 5, 10, and 20 μM ivermectin for another 6 h, and then they were tinted with oxidation of MitoSOX Red (Invitrogen, Carlsbad, CA) and DAPI, which is oxidized by superoxide in the mitochondria, emitting red fluorescence. Cultures were incubated for 10 min at 37°C and washed twice with warm HBSS. Production of mitochondrial ROS was analyzed using MitoSOX Red. The cell fluorescence was photographed by fluorescence microscopy.
Link to referenced study: Ivermectin has New Application in Inhibiting Colorectal Cancer Cell Growth
Ivermectin has New Application in Inhibiting Colorectal Cancer Cell Growth
Colorectal cancer (CRC) is the third most common cancer worldwide and still lacks effective therapy. Ivermectin, an antiparasitic drug, has been shown to possess anti-inflammation, anti-virus, and antitumor properties. However, whether ivermectin affects CRC is still unclear. The objective of this study was to evaluate the influence of ivermectin on CRC using CRC cell lines SW480 and SW1116. We used CCK-8 assay to determine the cell viability, used an optical microscope to measure cell morphology, used Annexin V-FITC/7-AAD kit to determine cell apoptosis, used Caspase 3/7 Activity Apoptosis Assay Kit to evaluate Caspase 3/7 activity, used Western blot to determine apoptosis-associated protein expression, and used flow cytometry and fluorescence microscope to determine the reactive oxygen species (ROS) levels and cell cycle. The results demonstrated that ivermectin dose-dependently inhibited colorectal cancer SW480 and SW1116 cell growth, followed by promoting cell apoptosis and increasing Caspase-3/7 activity. Besides, ivermectin upregulated the expression of proapoptotic proteins Bax and cleaved PARP and downregulated antiapoptotic protein Bcl-2. Mechanism analysis showed that ivermectin promoted both total and mitochondrial ROS production in a dose-dependent manner, which could be eliminated by administering N-acetyl-l-cysteine (NAC) in CRC cells. Following NAC treatment, the inhibition of cell growth induced by ivermectin was reversed. Finally, ivermectin at low doses (2.5 and 5 µM) induced CRC cell arrest. Overall, ivermectin suppressed cell proliferation by promoting ROS-mediated mitochondrial apoptosis pathway and inducing S phase arrest in CRC cells, suggesting that ivermectin might be a new potential anticancer drug therapy for human colorectal cancer and other cancers.
Introduction
Colorectal cancer (CRC) refers to malignant tumors in the ascending colon, transverse colon, descending colon, sigmoid colon, and rectum and is one of the most common malignant tumors worldwide. Among all malignant tumors globally, CRC ranks third in incidence and second in mortality (Siegel et al., 2020). CRC has caused a heavy economic burden on the country and individuals (Maida et al., 2017). At present, the treatment of CRC mainly adopts a comprehensive treatment based on surgery, combined with radiotherapy, chemotherapy, targeted therapy, and other treatments (Modest et al., 2019). However, due to the complicated mechanism of the occurrence, development, and metastasis of CRC, there is still a lack of specific drugs for CRC treatment.Ivermectin is a derivative of the 16-membered macrolide compound abamectin, which was first widely used in clinical practice as an antiparasitic drug (Laing et al., 2017). Ivermectin can increase the activity of γ-aminobutyric acid receptor or glutamate-chloride ion channel (Glu-Cl), increase the influx of chloride ions, and cause the cell membrane hyperpolarization, thereby blocking signal transmission between neurons and muscles (Martin et al., 2021), which exerts its antiparasitic effects. Ivermectin could be used, in addition to as an antiparasitic drug, as antiviral agents such as Flavivirus, HIV-1 virus, and SARS-CoV-2 virus (Mastrangelo et al., 2012; Wagstaff et al., 2012; Caly et al., 2020). Moreover, studies have shown that ivermectin has an inhibitory effect on various tumor cells and may be a potential broad-spectrum antitumor drug (Juarez et al., 2020). Juarez et al. (2020) have demonstrated that ivermectin is the most sensitive to breast cancer cells MDA-MB-231, MDA-MB-468, MCF-7, and ovarian SKOV-3; whereas ivermectin is the most nonsensitive to the prostate cancer cell line DU145. The induction of cell cycle arrest at G0/G1 mediates this effect of ivermectin on these sensitive cancer cells. Furthermore, ivermectin can inhibit the proliferation of cancer cells through p21-activated kinase 1 (PAK1)-induced autophagy, Caspase-dependent apoptosis, or immunogenic cell death regulate the signal pathways, including Hippo, Akt/mTOR, and WNT-TCF pathways to inhibit cancer cell proliferation (Liu et al., 2020). As known, ROS plays a vital role in the apoptosis caused by oxidative stress. ROS is a by-product of normal mitochondrial respiration. Stimuli such as infection, drought, cold, and ultraviolet light result in increased ROS in cells. Then, accumulative ROS could induce cells mitochondrial dysfunction and promote apoptosis in cells (Sinha et al., 2013). Evidence has shown that ivermectin-induced apoptosis is closely related to the production of ROS. Currently, there are few reports on the research of ivermectin in colorectal cancer.
Furthermore, new use of old drugs (that is, drug relocation) is a strategy for expanding old drugs and developing new uses, which has the advantages of low research and development cost and short development time (Pushpakom et al., 2019). Research on drug relocation of ivermectin is a shortcut to developing new antitumor drugs. Given this, we designed a study to explore the impact of ivermectin on the proliferation and apoptosis of CRC cells and the underlying mechanism.
Materials and Methods
Cell Culture
SW480 and SW1116 cells were acquired from ATCC and grown in DMEM medium (Biological Industries, Israel) supplemented with 10% FBS (Biological Industries, Israel), 1% penicillin/streptomycin (Coolaber, Beijing, China), and 2.5% HEPES buffer (Procell, Wuhan, China) in an incubator with a humidified air atmosphere of 5% CO2 at 37°C.Cell Viability Assay
Cells were seeded at a density of 1 × 104 cells/well in a 96-well plate. After being cultured overnight, cells were treated with ivermectin (Figure 1) (MCE Chemicals, Shanghai, China) at the indicated concentrations for 12, 24, or 36 h or cells were pretreated with N-Acetyl-l-cysteine (NAC, 5 mM) (Aladdin, Shanghai, China) for 1 h and then were cultured in ivermectin (20 μM) for 6 h. Then, 10 μL of CCK-8 solution was added to each well and incubated at 37°C for 1 h. The absorbance was detected at 450 nm by a microplate reader (SpectraMax i3x, Molecular Devices, United States). The cell viability was calculated as follows: (absorbance of drug-treated sample/absorbance of control sample) × 100.FIGURE 1

FIGURE 1. The chemical structure of ivermectin which is composed of ivermectin B1a (>90%) and ivermectin B1b.
Cell Morphology
Colorectal cancer cells were plated at 1 × 105 cells/well in twelve-well plates. After being cultured overnight, the cells were treated with ivermectin at the indicated dose for 24 h. Cell morphology was evaluated using an optical microscope.Flow Cytometry
Apoptosis was determined using the Annexin V-FITC/7-AAD Apoptosis Kit. Briefly, after colorectal cancer cells were exposed to ivermectin (0, 5, 10, and 20 μM) for 6 h, cells were centrifuged at 1,500 rpm for 5 min, washed, and suspended in PBS. Then, cells were stained with Annexin V-FITC and 7-AAD for 15 min. In addition, cells (1 × 106 cells/well) were cultured onto 6-well plates overnight and treated with indicated concentrations (0, 2.5, 5, and 10 μM) of ivermectin. Then, the cells were harvested and resuspended in PBS and fixed with 70% ethanol, and left at −20°C overnight. After 12 h of fixation, cells were centrifuged, washed, and resuspended in cold PBS. Then, the cells were added with 100 μL of RNase and incubated at 37°C for 30 min. The PI staining solution was then added and incubated at 4°C for 30 min. Cells were acquired by flow cytometry (FACSCanto Plus), and data were analyzed using Flowjo 10.0 software. The percentage of Q2 (early apoptosis, Annexin V+7-AAD-) plus Q3 (late apoptosis, Annexin V+7-AAD+) region was counted as the percentage of apoptosis cells.Caspase 3/7 Activity Assay
Caspase 3/7 assay was performed using the Caspase 3/7 Activity Apoptosis Assay Kit (Sangon Biotech, Shanghai, China). Briefly, after colorectal cancer cells (SW480 or SW1116) were treated with different concentrations of ivermectin (0, 5, 10, and 20 μM) for 6 h, we added 100 μL of Caspase 3/7 reagent into each well and mixed using a plate shaker. The Caspase 3/7 activity was then determined using a microplate reader (SpectraMax i3x). The Caspase 3/7 activity was expressed as a fold of the untreated control (Con) treatment.Western Blot Assay
After colorectal cancer cells (SW480 and SW1116) were treated with 0, 5, and 10 μM ivermectin for 6 h, they were collected, washed with PBS, and lysed with RIPA buffer. Protein quantification was determined using BCA Protein Assay Kit (EpiZyme Biotechnology, Shanghai, China). Equal amounts of protein were loaded onto an SDS-PAGE gel for electrophoresis and transferred to nitrocellulose. After blocking with 5% nonfat milk for 1 h, the membranes were incubated with the primary antibody [Bcl-2 (1:2000), Bax (1:2000), PARP (1:20,000) (all from Proteintech, Rosemont, IL, United States), and β-actin (1:5000) (Sigma-Aldrich)] on a 4°C shaker overnight. The membranes were then incubated with a secondary antibody for another 1 h at room temperature. A chemiluminescent gel imaging system detected the change in target protein expression (Universal Hood II, Bio-Rad, Hercules, CA, United States).ROS Measurement
For total ROS measurement, colorectal cancer cells (SW1116) were seeded (1 × 105 cells/well) in a 12-well plate and incubated overnight. Cells were treated with different concentrations of ivermectin for another 6 h, and then they were co-cultured with DCFH-DA (Invitrogen, Carlsbad, CA, United States) and DAPI (Biolegend, San Diego, CA, United States) for 20 min at 37°C in the dark. The cell fluorescence was photographed by fluorescence microscopy (OLYMPUS, Tokyo, Japan).For the mitochondrial ROS measurement, colorectal cancer cells (SW1116) were seeded in a 12-well plate and incubated overnight. After that, cells were treated with 0, 2.5, 5, 10, and 20 μM ivermectin for another 6 h, and then they were tinted with oxidation of MitoSOX Red (Invitrogen, Carlsbad, CA) and DAPI, which is oxidized by superoxide in the mitochondria, emitting red fluorescence. Cultures were incubated for 10 min at 37°C and washed twice with warm HBSS. Production of mitochondrial ROS was analyzed using MitoSOX Red. The cell fluorescence was photographed by fluorescence microscopy.
<This is the end of the first part of referenced article. You can find the complete piece at the referenced link (above)>
energy_wave
Has No Life - Lives on TB
So, do you take it orally or up the back door?
Knoxville's Joker
Has No Life - Lives on TB
Some cancers are fungus. Others are results of parasitic infections...
psychgirl
Has No Life - Lives on TB
I’ve heard this too. But not read anything myself.Some cancers are fungus. Others are results of parasitic infections...
Knoxville's Joker
Has No Life - Lives on TB
That is mainly because any findings in that area get retconned shortly after it comes out...I’ve heard this too. But not read anything myself.
bw
Fringe Ranger
Diatomaceous earth is a mechanical insecticide and abrasive. It has no chemical payload, so it's not getting beyond your digestive system.Would it be a stretch to take Diatomtious Earth for cancer prevention? At least for the parasitical side...
This sounds like somebody would have to take a low dose of ivermectin daily to make a difference. Any suggestions for a daily dose? Six months ago, I had three polyps removed during a colonoscopy, one of which was pre-cancerous. If I had waited another year, I would have had colon cancer. Doctor says I have to do a colonoscopy every year now, so the issue of daily dosing interests me.
